valence electron of aluminum|number of valence electrons list : Pilipinas The valence electron of aluminum is +3, according to the table of element valences. This is the most common and maximum valence value for aluminum, which has a filled 3rd . This website is operated by Unibet/Betchoice Corporation Pty Ltd. ABN 71 121 382 607 , whose registered office is Fannie Bay Racecourse, Dickward Drive, FANNIE BAY NT 0820 Licensed and regulated by Australia's Northern Territory Government.
PH0 · valence electrons chart
PH1 · valence electrons calculator
PH2 · valence electron configuration calculator
PH3 · number of valence electrons list
PH4 · how to find valence electrons
PH5 · how many valence electrons in titanium
PH6 · how many electrons does aluminum have
PH7 · aluminum electron configuration shells
PH8 · Iba pa
Here, in the Alliance of the Two Hearts, one commits himself to love one another unto death. Thus healing of brokenness occurs. The Alliance of the holy Family International was born in response to Pope John Paul II invitation to make the Alliance of the Two Hearts known, understood and loved throughout the world. (Cf.
valence electron of aluminum*******Learn how to calculate the valence electrons of aluminum (Al) by following a few steps. The valence electrons are the total number of electrons in the last shell after the electron configuration of aluminum. The valence electrons of aluminum are three and its .
Valence electrons; 1: Hydrogen (H) 1: 2: Helium (He) 2: 3: Lithium (Li) 1: 4: Beryllium (Be) 2: 5: Boron (B) 3: 6: Carbon (C) 4: 7: Nitrogen (N) 5: 8: Oxygen (O) 6: 9: Fluorine (F) 7: .The valence electron of aluminum is +3, according to the table of element valences. This is the most common and maximum valence value for aluminum, which has a filled 3rd .There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum has in its .Write the configuration of Aluminum, assuming that it has lost its valence electrons. What happens to aluminum when it reacts with chlorine? Balance these equations: a) 2Al(s) .
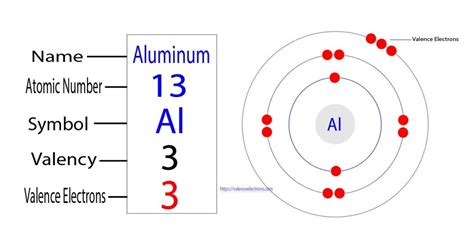
Aluminum Valence Electrons: Aluminum is the chemical element denoted with a symbol (Al) and with an atomic number 13. Aluminum is in silvery-white color metal. Al is metal .valence electron of aluminum number of valence electrons listExample \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur .
Explanation: The electron configuration for Aluminum is. 1s22s22p63s23p1. The first 10 electrons are in the filled first and second shells. This leaves only three electrons in the .
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around .The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different .valence electron of aluminum Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Therefore, the valence electrons of aluminum are three. The last shell of aluminum has three unpaired electrons, so the valency of aluminum is 3. The last electron of aluminum enters the p-orbital. Therefore, it’s a p . The valence electron configuration for aluminum is 3s 2 3p 1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber \nonumber \] The valence electron configuration for selenium is 4s 2 4p 4.
The shorthand electron configuration for Aluminum is [Ne] 3s 2 3p 1. The electron configuration for the Aluminum ion (Al 3+ ) is 1s 2 2s 2 2p 6. The number of valence electrons available for the Aluminum atom is 3. Aluminum is situated in Group 13th or 3A and has an atomic number of 13. Slater's Rules. Step 1: Write the electron configuration of the atom in the following form: (1s) (2s, 2p) (3s, 3p) (3d) (4s, 4p) (4d) (4f) (5s, 5p) . . . Step 2: Identify the electron of interest, and ignore all electrons in higher groups (to the right in the list from Step 1).These do not shield electrons in lower groups; Step 3: Slater's Rules is now .The correct option is D 3. Valency is the combining capacity of an atom. It depends on the no. of valence electrons. Atomic number. of aluminium is 13. Thus, it contains 13 protons and 13 electrons. Electronic configuration of aluminium (Al) is 2,8,3. Valency = no. of valence electrons (n), if n<4. Thus, valency of aluminum is 3.
Method 2: From the Electron Configuration. If you want to find the valence electrons of aluminum from its electron configuration, then you should know its electron configuration first. Now there are many methods to write the electron configurations, but here I will show you the easiest method, i.e by using Aufbau principle. Aufbau principle . Le numéro atomique de l’aluminium est 13 selon le tableau périodique. Cela signifie qu’un atome d’aluminium (Al) contient un nombre total de treize électrons. La valence est une caractéristique numérique de la capacité des atomes d’un élément donné à se lier avec d’autres atomes.
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable . A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. . (since metals are located on the left side of the periodic table and do not have many electrons in their valence shells). The theory must also account for all of a metal's unique chemical and physical properties.How many electrons does aluminum-27 have in its valence electron shell? This question is asking us to determine the number of electrons found in the valence electron shell of the aluminum-27 isotope. First of all, the valence electron shell is the outermost electron shell of an atom. And the electrons found in this shell are called valence .number of valence electrons listHow many electrons does aluminum-27 have in its valence electron shell? This question is asking us to determine the number of electrons found in the valence electron shell of the aluminum-27 isotope. First of all, the valence electron shell is the outermost electron shell of an atom. And the electrons found in this shell are called valence .
Element Aluminium (Al), Group 13, Atomic Number 13, p-block, Mass 26.982. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each .Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum (Al), with an atomic number of 13, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1. In this configuration, the outermost shell is the third shell (3s 2 3p 1 ), which contains 3 .
The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. . This means that B has two valence electrons in 3s (3s 2). Answer: 3s 2. Element C is located in Period 5, the 1st position in 5s-block).As reductants, the group 13 elements are less powerful than the alkali metals and alkaline earth metals. Nevertheless, their compounds with oxygen are thermodynamically stable, and large amounts of energy are needed to isolate even the two most accessible elements—boron and aluminum—from their oxide ores. Figure 21.6.1 21.6. 1: Borax .Since aluminium has three valence electrons, three fluorine atoms can bond. AlF 3 is the chemical formula. Aluminium trichloride. Chlorine (Cl) can also form a bond with aluminium (Al). Aluminium has three extra electrons that can easily be used by chlorine atoms to bond. The formula of the compound is AlCl 3. Aluminium Phosphide
The electron configuration of aluminum is [ Ne] 3s 2 3p 1. In the above electron configuration, the highest energy level (3) is marked with green color. The 3 rd energy level contains 3s and 3p subshells. There are 2 electrons in the 3s subshell and 1 electron in the 3p subshell. So aluminum has a total of 2 + 1 = 3 valence electrons.
WOW Vegas Casino Review. Over 500+ games to choose from. Great bonuses in US. Easy to use website. Copy promo code: SBRBONUS. Play Now. #3. 245,000 Gold Coins + 117.5 Free Sweepstakes Coins..
valence electron of aluminum|number of valence electrons list